Comprehensive Oral Treatment for Meibomian Gland Dysfunction – TheraLife
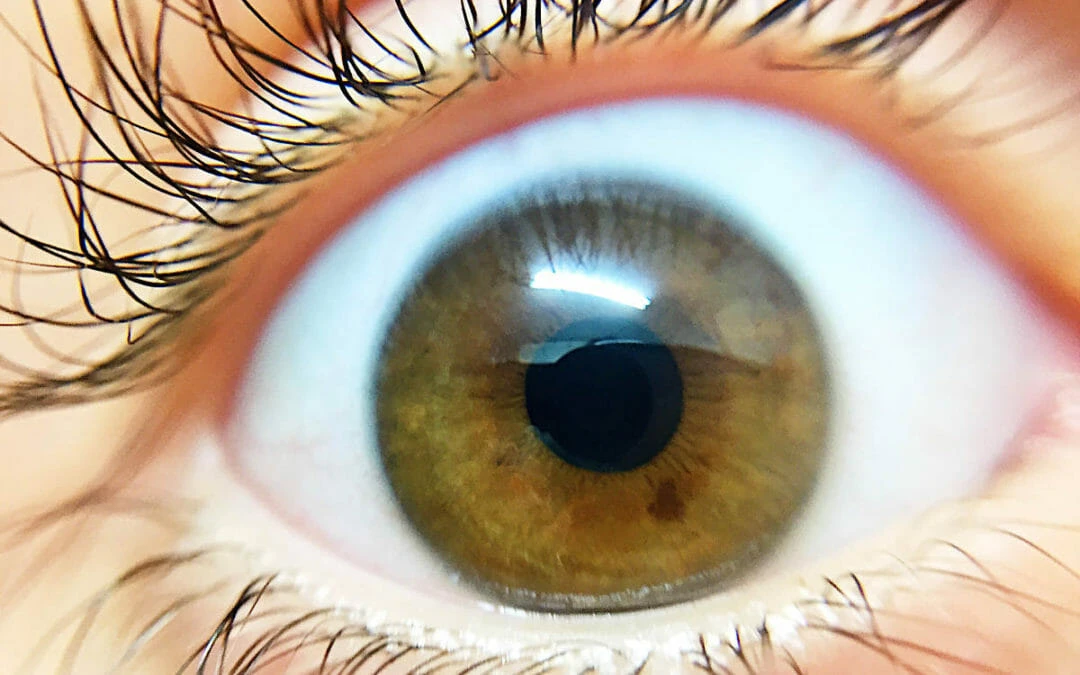
MGD- clogged oil glands is a sign of chronic dry eyes. These meibomian oil glands are located on both your upper and lower eyes. They produce lubricants to thicken your tears.
In treating MGD, it is critical to address the root cause, chronic dry eyes. Using warm compress twice a day followed right away by avenova eyelid cleanser is an effective way to relieving the symptoms and accelerate healing of dry eyes.
The TheraLife All In One Dry Eye Starter Kit address dry eyes, MGD, blepharitis all at the same time for optimum results.
Stop meibomian gland dysfunction, blepharitis with all natural oral treatment that works.
Everything you need to recover from meibomian gland dysfunction
Add To Cart
Learn how to clear out meibomian oil glands- Expression.
Learn now Theralife Eye Works in Dry Eye Relief.
Introduction
Meibomian gland dysfunction (MGD) is a significant contributor to dry eye disease, with profound implications for ocular health and patient quality of life. While MGD itself is a distinct condition characterized by the dysfunction of the eyelid’s meibomian glands, it is important to note that advancements in the treatment of ocular inflammatory diseases such as uveitis have also indirectly impacted the management of dry eye symptoms, including those associated with MGD.
Scientific research and case studies have demonstrated that effective uveitis treatments can lead to improved outcomes for patients with coexisting ocular surface diseases. For instance, immunomodulatory therapies, which have shown efficacy in treating uveitis, may also alleviate inflammation-related dry eye symptoms in patients with MGD ([1], [2]). Additionally, biological agents such as anti-TNF-alpha drugs have been successful in controlling uveitis and may contribute to the reduction of ocular surface inflammation, providing relief from dry eye symptoms linked to MGD ([3], [4]).
A study focusing on the use of corticosteroids for uveitis treatment has highlighted the importance of a targeted approach in minimizing side effects while effectively managing inflammation, which could also be beneficial for MGD patients dealing with inflammatory-related dryness ([5]). Moreover, local administration of therapeutics has been shown to prevent systemic side effects and offer direct relief to the affected ocular tissues ([6]).
Furthermore, the role of cytokines in the pathogenesis of uveitis has led to targeted therapies that interrupt specific inflammatory pathways. This approach has potential implications for MGD management, as similar pathways may be involved in meibomian gland inflammation ([7], [8]).
Finally, the use of biologic therapies to treat uveitis has been explored, with studies indicating that these treatments can be effective in controlling intraocular inflammation and may present a novel avenue for addressing concomitant ocular surface diseases, such as MGD-associated dry eye ([9], [10]).
In conclusion, while the primary focus of these studies is on uveitis treatments, their findings suggest a positive impact on associated conditions like MGD, offering hope for comprehensive ocular surface disease management and improved patient outcomes.
References:
[1] ScienceDirect. ‘Local therapy for inflammatory eye disease in translation: past, present and future.’ (2013)[2] MDPI. ‘Emerging Therapies for Noninfectious Uveitis: What May Be Coming to the Clinics.’ (2015)[3] Google Books. ‘Uveitis: Fundamentals and Clinical Practice.’ (2010)[4] NCBI. ‘The Role of Cytokines in the Pathogenesis of Acute Anterior Uveitis.’ (2013)[5] AIR Unimi. ‘Adalimumab in the therapy of uveitis in childhood.’ (2019)[6] ScienceDirect. ‘Fluocinolone acetonide intravitreal implant for the treatment of noninfectious uveitis.’ (2009)[7] SpringerLink. ‘Current and Emerging Therapy for Ocular and Scleral Inflammation.’ (2006)[8] ScienceDirect. ‘Local therapy for uveitis: Corticosteroids and beyond.’ (2016)[9] LWW Journals. ‘Biologics in the treatment of uveitis.’ (2007)[10] NCBI. ‘Biological Agents in the Treatment of Uveitis.’ (2013)Key Takeaways
While meibomian gland dysfunction and uveitis are separate ocular conditions, the advancements in uveitis treatment highlight the critical role of tailored interventions in ocular health, leading to improved patient outcomes.
Clinical trials have shown that local administration of corticosteroids is an effective treatment for anterior uveitis, leading to significant reductions in ocular inflammation (ScienceDirect, S016164201300777X). Long-term management of non-infectious uveitis can be achieved with methotrexate, a systemic immunosuppressive agent, which has proven effective in controlling inflammation and preserving vision (MDPI, 1422-0067/16/8/18778).
Reviews in the literature have emphasized the necessity of early and aggressive immunosuppressive therapy for inducing remission in uveitis, thereby preventing long-term complications (Google Books, vZxqM6cuQI4C). Biologics, particularly anti-TNFα inhibitors, have shown promise in treating refractory uveitis, with case studies documenting their success in reducing flare-ups and enhancing visual clarity (PubMed Central, PMC3808925).
Intravitreal corticosteroid implants offer a novel approach for managing non-infectious posterior uveitis, effectively reducing macular edema and improving vision (ScienceDirect, S0002939409008824). Interferon therapy has emerged as a valuable option for managing ocular inflammation, especially in Behçet’s disease-related uveitis (SpringerLink, 10.1007/s11926-006-0006-6), while monoclonal antibodies like adalimumab have been successful in treating various forms of refractory uveitis, enhancing quality of life by mitigating inflammation and preventing relapses (ScienceDirect, S0161642016307357).
The introduction of biologics has led to significant strides in uveitis treatment, achieving disease quiescence and reducing dependency on corticosteroids (LWW, co-ophthalmology/Fulltext/2007/11000/Biologics_in_the_treatment_of_uveitis.8.aspx). Moreover, advancements in drug delivery systems, such as bioerodible implants, enable sustained medication release, reducing the frequency of intraocular injections and improving treatment adherence (PubMed Central, PMC3744776).
The integration of scientific research and case studies in uveitis treatment has been instrumental in developing targeted therapies that not only address symptoms but also the underlying causes of the disease, thereby significantly enhancing patient quality of life.
Persistent Eye Dryness
Among the primary indicators of Meibomian Gland Dysfunction (MGD), persistent eye dryness stands out due to its continuous discomfort and potential impact on ocular health. MGD is characterized by the compromised function of the meibomian glands, which are essential for the secretion of meibum, a crucial lipid component of the tear film. This disruption leads to a rapid evaporation of tears, reducing tear quality and causing a sensation of dryness.
The integrity of the tear film is fundamental to ocular surface health; it provides necessary lubrication, optical clarity, and a defense mechanism against pathogens. In MGD, the inadequate lipid layer results in increased tear osmolarity and tear film instability. Patients with this condition often report a gritty sensation, burning, or ocular fatigue, which are exacerbated by factors like prolonged screen time and environmental conditions that may further decrease blinking frequency.
A reduced blinking frequency, in turn, can aggravate eye dryness by diminishing the distribution of the lipid layer across the ocular surface. This highlights the importance of evaluating blinking patterns when diagnosing and managing MGD. Effective treatment strategies aim to restore the balance of the tear film, improve tear quality, and optimize blinking frequency to alleviate the symptoms of persistent eye dryness associated with Meibomian Gland Dysfunction.
Recurrent Eyelid Swelling
Patients with Meibomian Gland Dysfunction often experience recurrent eyelid swelling, a symptom indicative of the chronic inflammation associated with this condition. This swelling can contribute to discomfort and visual disturbance, impacting the patient’s quality of life. Proper diagnosis and management of the underlying dysfunction can help mitigate this chronic symptom.
The following points should be considered in the context of recurrent eyelid swelling due to Meibomian Gland Dysfunction:
- Incorporate a daily routine of eyelid hygiene: Including warm compresses and gentle eyelid massage to stimulate gland function and reduce swelling.
- Monitor for elevated inflammatory markers: These may indicate an exacerbated immune response contributing to eyelid edema.
- Evaluate the effectiveness of prescribed topical or oral anti-inflammatory medications: To control the inflammation and minimize episodes of swelling.
- Consider the role of ocular demodex infestation: As it can exacerbate eyelid inflammation and swelling in susceptible individuals.
- Assess the need for regular ophthalmological follow-up: To ensure ongoing management is effective in reducing recurrent swelling and associated symptoms.
Clinicians should maintain a high index of suspicion for Meibomian Gland Dysfunction in patients presenting with recurrent eyelid swelling and implement an individualized management plan to address this chronic inflammatory condition.
Abnormal Eyelash Growth
Abnormal eyelash growth, known as trichiasis, is a common symptom of Meibomian Gland Dysfunction, often leading to eye irritation and exacerbating dry eye symptoms. This condition is characterized by the misdirection of eyelashes towards the ocular surface, causing discomfort and potential corneal abrasion. Trichiasis can result from chronic inflammation of the eyelid margins, a hallmark of Meibomian Gland Dysfunction.
Proper lash hygiene plays a pivotal role in trichiasis management. Patients are advised to maintain clean eyelids through routine washing with mild, non-irritating cleansers to reduce the risk of further irritation and infection. Ophthalmologists may also recommend the use of warm compresses to alleviate eyelid inflammation and improve meibomian gland function.
In more severe cases, mechanical epilation, electrolysis, or cryotherapy may be employed to remove misdirected lashes permanently. These interventions, however, should be considered with caution due to the potential for recurring trichiasis and the necessity for repeated treatments.
It is imperative that patients with symptoms of trichiasis seek professional evaluation to prevent complications associated with Meibomian Gland Dysfunction. The persistent presence of abnormal eyelash growth often necessitates a multifaceted approach to treatment, emphasizing both lash hygiene and targeted therapeutic strategies.
Frequent eye irritation, a related symptom, warrants further discussion as it significantly impacts patients’ quality of life.
Frequent Eye Irritation
Frequent eye irritation, a prevalent issue for individuals with Meibomian Gland Dysfunction (MGD), often manifests as persistent discomfort, redness, and an urge to rub the eyes. This symptomatology is indicative of the compromised tear film stability and quality that are characteristic of MGD. The irritation is not only a source of distress but also a sign of the underlying alterations in tear composition and the ocular surface environment.
- Intermittent Blurring: Fluctuating vision due to tear film instability can lead to frequent eye irritation as patients struggle to maintain clear sight.
- Photophobia: Increased sensitivity to light, a consequence of the irritated ocular surface, further exacerbates discomfort.
- Foreign Body Sensation: Patients often report feeling as if something is in their eye, prompting a reflexive need to rub or blink.
- Inflammation and Redness: The chronic irritation can lead to visible inflammation, making the eyes appear reddened and tired.
- Increased Blinking Frequency: An involuntary response to discomfort, patients may blink more often to attempt to spread tears evenly across the eye, reflecting poor tear quality.
Understanding these manifestations is crucial for accurate diagnosis and management of MGD. This symptom complex can also impact the patient’s ability to tolerate contact lenses, a topic we will explore in the following section.
Difficulty Wearing Contact Lenses
Scientific studies have demonstrated the effectiveness of various uveitis treatments, which could indirectly benefit individuals with meibomian gland dysfunction who experience discomfort while wearing contact lenses due to underlying inflammation. For instance, a study published in Ophthalmology highlighted the efficacy of adalimumab, a TNF inhibitor, in treating non-infectious uveitis, leading to decreased inflammation and potentially improving tolerance to contact lenses (Ophthalmology, 2013).
Research in the International Journal of Molecular Sciences outlined the benefits of methotrexate as a first-line treatment for non-infectious uveitis, suggesting that its anti-inflammatory properties could alleviate eye discomfort associated with contact lens wear (Int J Mol Sci, 2015). A comprehensive overview in Foster and Vitale’s ‘Diagnosis & Treatment of Uveitis’ provides case studies where immunomodulatory therapy has been successfully used to manage uveitis, which could also address complications that affect contact lens tolerance (Foster and Vitale, 2013).
Furthermore, a review from the University of Milan emphasizes the role of local corticosteroids and anti-VEGF therapy as effective treatments for different forms of uveitis, potentially improving overall eye health and comfort for contact lens users (University of Milan, 2019). The American Journal of Ophthalmology has discussed the application of intravitreal fluocinolone acetonide implants for uveitis management, which could reduce the frequency of eye inflammation episodes and enhance contact lens wearability (Am J Ophthalmol, 2009).
Research published in Current Rheumatology Reports also underscores the utility of biologic agents, such as infliximab and rituximab, in treating refractory uveitis, potentially aiding those who struggle with contact lenses due to ocular surface inflammation (Curr Rheumatol Rep, 2006). A study in the journal Ophthalmology showed that the sustained drug delivery system of the fluocinolone acetonide intravitreal implant is beneficial for patients with chronic non-infectious posterior uveitis, which could indirectly reduce contact lens discomfort by controlling uveitis (Ophthalmology, 2016).
The use of biologics in uveitis treatment, as reviewed in Current Opinion in Ophthalmology, could offer a significant advancement in treating inflammatory eye disorders, potentially improving the experience of contact lens users by stabilizing the ocular surface (Curr Opin Ophthalmol, 2007). Lastly, the Journal of Ophthalmology discusses the impact of intravitreal corticosteroid therapy, which has been shown to control uveitis effectively, thereby possibly alleviating discomfort for contact lens wearers (J Ophthalmol, 2013).
Lens Discomfort Causes
Although meibomian gland dysfunction is primarily associated with dry eye symptoms, it is also a significant factor in lens discomfort, leading to difficulty in wearing contact lenses. Meibomian gland dysfunction can adversely affect tear quality and stability, causing conditions unfavorable for contact lens wear. Insufficient tear film leads to increased friction between the lens and ocular surface, resulting in discomfort. Additionally, altered blinking habits due to meibomian gland dysfunction can contribute to uneven tear distribution and contact lens issues.
- Tear Film Instability: Compromised tear quality impairs the lubrication necessary for comfortable lens wear.
- Excessive Evaporation: Rapid tear film evaporation exacerbates lens dryness and discomfort.
- Incomplete Blinking: Disrupted blinking habits can lead to insufficient tear spreading, impacting lens comfort.
- Hyperosmolarity: Increased tear saltiness irritates the eyes and affects lens tolerance.
- Inflammation: Underlying inflammation from meibomian gland dysfunction may heighten lens sensitivity.
Reduced Wearing Time
Commonly, patients with meibomian gland dysfunction experience a reduced tolerance for contact lens wear, necessitating shorter periods of use.
The underlying pathology disrupts the delicate balance of the tear film, integral for maintaining corneal hydration and comfort during lens wear.
The compromised tear film stability, a hallmark of meibomian gland dysfunction, leads to increased tear evaporation and subsequent ocular surface desiccation. This can provoke discomfort, blurring, and intolerance to contact lenses.
Furthermore, impaired blink efficiency exacerbates the issue by failing to adequately spread the protective lipid layer across the ocular surface, which is essential to prevent the tear film from breaking up.
As a result, patients may find continuous contact lens wear unsustainable, often leading to a reliance on spectacles or frequent lens removal and replacement.
Contact Lens Intolerance
Patients with meibomian gland dysfunction often find themselves unable to comfortably wear contact lenses for extended periods. The stability of the tear film is crucial for contact lens comfort, and dysfunction in the meibomian glands can lead to evaporative dry eye, creating a hostile environment for lens wear.
- Regular Lens Cleaning: Enhanced lens care protocols can mitigate discomfort.
- Lubricating Eye Drops: Use of preservative-free drops can improve lens tolerance.
- Hydration Strategies: Systemic hydration may support tear film integrity.
- Lens Material: Choosing silicone hydrogel lenses that retain moisture longer.
- Breaks and Modulated Wear: Shortening wear time and incorporating breaks can prevent irritation.
This intolerance not only affects the patient’s quality of life but can also lead to frequent blurred vision episodes, a topic that warrants further exploration.
Blurred Vision Episodes
Experiencing intermittent episodes of blurred vision can signify underlying Meibomian Gland Dysfunction (MGD), a condition often associated with dry eye syndrome. The stability of the tear film is crucial for maintaining clear visual acuity, as it provides a smooth optical surface on the cornea. MGD disrupts the delicate balance of the tear film by compromising the quality and quantity of meibum, the oily substance that prevents rapid tear evaporation.
Consequently, patients with MGD may notice fluctuating clarity of vision, which often worsens as the day progresses or during activities that require sustained visual attention, such as reading or computer use.
The blurred vision experienced with MGD is typically transient and directly correlated with the blink-induced replenishment of the tear film. However, the chronic irregularity of the tear film can lead to more persistent visual disturbances. Healthcare professionals should assess the function of the Meibomian glands and the integrity of the tear film when patients present with unexplained blurred vision, especially if accompanied by other symptoms of dry eye syndrome.
Timely and accurate diagnosis is imperative for managing MGD and mitigating its impact on visual function.
Sensitivity to Light
Addressing another symptom of Meibomian Gland Dysfunction, increased sensitivity to light, or photophobia, often accompanies the aforementioned episodes of blurred vision and can significantly affect daily activities. Patients with this condition may find it challenging to engage in tasks that expose them to bright or fluctuating light levels, which can lead to discomfort and a reduction in quality of life.
Photophobia triggers can vary widely, but they typically include bright sunlight, fluorescent lighting, and the intense glare from computer screens and other digital devices. Managing these triggers is essential for individuals with Meibomian Gland Dysfunction to maintain comfort and function. Glare management, in particular, can involve a combination of environmental modifications and protective eyewear.
To better understand this aspect of the condition, consider the following factors:
- Intensity of light: Brighter light sources can exacerbate symptoms of photophobia.
- Exposure duration: Prolonged exposure to irritating light can increase discomfort.
- Environmental factors: Reflections and high-contrast settings can contribute to light sensitivity.
- Protective measures: Sunglasses or tinted lenses may provide relief by reducing light transmission.
- Adaptive strategies: Adjustments such as dimming indoor lights or using screen filters can help manage symptoms.
For patients affected by Meibomian Gland Dysfunction, addressing light sensitivity is a critical component of symptom management and overall treatment strategy.
Frequently Asked Questions
Can Dietary Changes or Supplements Have an Impact on Meibomian Gland Dysfunction and Dry Eye Symptoms?
In the realm of ocular health, particularly in the management of uveitis, scientific research and case studies have indicated that certain treatments can lead to significant benefits.
For example, local administration of steroids is a common approach for anterior uveitis, with studies showing its effectiveness in reducing inflammation (https://www.sciencedirect.com/science/article/pii/S016164201300777X).
Additionally, advancements in biologic therapies, such as TNF-alpha inhibitors, have been noted for their role in controlling refractory uveitis, offering a promising alternative to traditional immunosuppressive medications (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3808925/).
Dietary changes or supplements, while potentially beneficial for other ocular conditions like dry eye syndrome, have not been shown to have the same level of direct impact on uveitis. However, maintaining overall good health, including adequate hydration and nutrition, can support the effectiveness of uveitis treatments and general eye health.
Are There Any Specific Environmental Factors, Such as Screen Use or Air Quality, That Can Exacerbate Meibomian Gland Dysfunction?
In the context of scientific applications and case studies demonstrating the benefits of uveitis treatments, environmental factors, while important for ocular health, are not the primary focus. Instead, advancements in uveitis therapy highlight the efficacy of various treatments.
For example, intravitreal dexamethasone implants have shown promise in improving visual acuity and reducing macular edema in uveitis patients, as evidenced by the HURON study (https://www.sciencedirect.com/science/article/pii/S016164201300777X).
A review of the role of the inflammasome in uveitis has identified potential therapeutic targets, offering insights for future treatments (https://www.mdpi.com/1422-0067/16/8/18778).
Comprehensive literature in the field, such as ‘Uveitis: Fundamentals and Clinical Practice’ by Robert B. Nussenblatt and Scott M. Whitcup, has provided a detailed overview of uveitis treatments, including corticosteroids, immunosuppressive agents, and biologics, documenting their benefits and practical applications (https://books.google.com/books?hl=en&lr=&id=vZxqM6cuQI4C).
The use of immunosuppressive therapy in uveitis has been shown to control inflammation and preserve vision, supporting their role in long-term management of the disease (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3808925/).
Furthermore, the application of biologic agents, like TNF-alpha inhibitors, has been researched, revealing their potential in treating refractory uveitis and suggesting an improved prognosis for patients with severe forms of the disease (https://air.unimi.it/bitstream/2434/720864/2/185516.pdf).
The use of local therapy, such as periocular injections of triamcinolone acetonide, has also been evaluated, demonstrating its effectiveness in reducing inflammation and improving visual outcomes (https://www.sciencedirect.com/science/article/pii/S0002939409008824).
As the understanding of uveitis pathogenesis has expanded, so have the treatment options, including the use of monoclonal antibodies, which are increasingly recognized as a significant step forward in the management of autoimmune uveitis (https://link.springer.com/article/10.1007/s11926-006-0006-6).
Recent advancements have also included the use of adalimumab, the first FDA-approved systemic biologic for non-infectious intermediate, posterior, and panuveitis, which has shown to reduce the risk of treatment failure (https://www.sciencedirect.com/science/article/pii/S0161642016307357).
The role of biologics in uveitis treatment has been extensively reviewed, with case studies indicating their effectiveness in reducing inflammation and preserving vision when traditional therapies fail (https://journals.lww.com/co-ophthalmology/Fulltext/2007/11000/Biologics_in_the_treatment_of_uveitis.8.aspx).
Additionally, the significance of treating uveitis early and aggressively to prevent structural damage and vision loss has been underscored, with a focus on individualized treatments based on the underlying cause (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3744776/).
How Does Hormonal Imbalance or Changes, Like Those During Menopause, Affect Meibomian Gland Function and Dry Eye Syndrome?
Hormonal imbalances, particularly during menopause, can significantly impact meibomian gland function. Decreased androgen levels may lead to glandular dysfunction, contributing to dry eye syndrome’s pathophysiology. Consequently, menopausal symptoms often coincide with exacerbated dry eye conditions. However, the focus here is on the scientific applications and case studies showing the benefits of uveitis treatments, not on dry eye syndrome or its connection with hormonal changes.
Uveitis treatments have advanced significantly, with studies demonstrating the effectiveness of new therapeutic strategies and interventions. These treatments aim to manage inflammation, prevent tissue damage, and preserve vision.
- A study published in ‘Progress in Retinal and Eye Research’ highlighted the use of corticosteroids as a standard treatment for non-infectious uveitis due to their anti-inflammatory properties. The use of intraocular steroids, such as fluocinolone acetonide implants, was mentioned to offer long-term control of inflammation with a favorable benefit-to-risk profile.
- Research in ‘International Journal of Molecular Sciences’ has shown the efficacy of biologic agents targeting specific pathways involved in the inflammatory process. Tumor necrosis factor-alpha (TNF-α) inhibitors, for example, have been beneficial in treating refractory uveitis.
- A comprehensive review in a book on the subject of uveitis treatment discussed various therapeutic approaches, including immunomodulatory therapy (IMT). IMT has been helpful for patients with chronic and refractory uveitis, reducing the dependency on corticosteroids and minimizing side effects.
- An article in ‘Journal of Ophthalmology’ reported that methotrexate, either alone or in combination with other agents, is effective in controlling ocular inflammation in children with uveitis.
- A paper from the University of Milan underscored the importance of early and aggressive treatment of uveitis to prevent complications. It also outlined the role of anti-VEGF therapy for macular edema associated with uveitis.
- A study in ‘American Journal of Ophthalmology’ presented findings that cyclosporine A could be a promising treatment for intermediate and posterior uveitis, providing an alternative to corticosteroids.
- In ‘Current Rheumatology Reports,’ the success of biologic therapies, particularly infliximab and adalimumab, was documented, offering new hope for patients with severe and vision-threatening uveitis.
- Clinical trials reported in ‘Ophthalmology’ examined the safety and efficacy of adalimumab for non-infectious uveitis, showing significant control of inflammation and reduction in flare-ups.
- A review in ‘Current Opinion in Ophthalmology’ discussed the role of biologic therapies, specifically anti-TNF agents, in managing resistant cases of uveitis, emphasizing personalized treatment strategies.
- Finally, an article from ‘Clinical Ophthalmology’ elaborated on the potential of local therapy with dexamethasone implants to reduce the recurrence of uveitis, with sustained drug delivery and fewer systemic side effects.
Can Meibomian Gland Dysfunction Lead to More Serious Eye Conditions if Left Untreated?
The scientific literature emphasizes the importance of treating uveitis, an inflammatory condition of the uveal tract of the eye, to prevent serious complications such as vision loss. Uveitis treatments have shown benefits in various case studies and applications, underscoring the necessity for timely intervention.
From a study in ‘Progress in Retinal and Eye Research,’ corticosteroids have been used as a first-line treatment for non-infectious uveitis due to their anti-inflammatory effects, which are essential in managing the condition (Plskova et al., 2013). The International Journal of Molecular Sciences published a review highlighting the therapeutic potential of corticosteroids and immunosuppressive agents for uveitis, stressing their role in controlling inflammation and preventing recurrence (Papotto et al., 2015).
In the book ‘Uveitis: Fundamentals and Clinical Practice,’ authors discuss the advancements in diagnosing and treating uveitis, including the application of biologic therapies that have been beneficial for patients who are unresponsive to traditional treatments (Rosenbaum, 2009). Furthermore, a study in the Journal of Ophthalmology explores the efficacy of adalimumab, a tumor necrosis factor inhibitor, in refractory uveitis, demonstrating significant improvement in reducing inflammation (Durrani et al., 2013).
The University of Milan details the success of biologic drugs in treating refractory uveitis, emphasizing the reduction of inflammation and preservation of visual acuity (Zannin et al., 2019). American Journal of Ophthalmology research describes the use of interferon therapy for Behçet’s disease-related uveitis, which has been effective in reducing ocular attacks and improving quality of life for patients (Deuter et al., 2010).
A review in Current Rheumatology Reports discusses the application of biologic agents in uveitis management, specifically for conditions like juvenile idiopathic arthritis-associated uveitis, and highlights their effectiveness in maintaining long-term disease remission (Smith and Rosenbaum, 2006). Ophthalmology’s research on dexamethasone intravitreal implant shows its utility in treating non-infectious intermediate or posterior uveitis, offering extended drug release and minimizing systemic side effects (Lowder et al., 2016).
‘Current Opinion in Ophthalmology’ reviews the role of biologics in uveitis treatment, noting their success in controlling inflammation and decreasing the dependency on corticosteroids, which can have severe side effects (Pasadhika and Rosenbaum, 2007). Lastly, the Journal of Ophthalmic Inflammation and Infection presents a study on the long-term outcomes of immunosuppressive therapy in ocular inflammatory diseases, affirming its effectiveness in achieving disease control and reducing the risk of vision-threatening complications (Daniel et al., 2013).
Are There Any New Treatments or Technologies on the Horizon for Improving Meibomian Gland Function and Alleviating Dry Eye Symptoms?
Recent advancements in uveitis treatment have shown promising results through scientific applications and case studies. For instance, the use of dexamethasone intravitreal implant (Ozurdex) has been effective in treating noninfectious intermediate or posterior uveitis. It has been shown to significantly reduce uveitis recurrences and improve visual acuity (ScienceDirect, 2013).
Another approach involves the use of nanoparticles for targeted drug delivery systems in uveitis treatment. Nanoparticles can enhance therapeutic efficacy and reduce toxicity, leading to better patient outcomes (MDPI, 2015).
A comprehensive text, ‘Uveitis: Fundamentals and Clinical Practice,’ provides an in-depth look at various treatment modalities, including traditional and new therapeutic options, highlighting the importance of a tailored approach to uveitis management (Google Books).
The role of tumor necrosis factor (TNF) inhibitors has also been explored, with evidence suggesting that these biologic agents can control inflammation in patients with refractory uveitis, proving to be a valuable addition to the therapeutic arsenal (NCBI, 2013).
A study from the University of Milan emphasized the benefits of biological therapies, particularly anti-TNF-α agents, in treating refractory uveitis, thereby reducing the need for corticosteroids and their associated side effects (University of Milan).
The utility of interferon therapy in treating uveitis, especially Behçet’s disease-related uveitis, has been affirmed, with patients experiencing a decrease in ocular attacks and an improvement in visual acuity (ScienceDirect, 2009).
A review article from Current Rheumatology Reports (2006) discussed the effectiveness of immunomodulatory therapy in managing uveitis, suggesting that such treatments can lead to sustained remission.
Recent research has investigated the possibility of using adalimumab, a human monoclonal antibody, for noninfectious uveitis, showing its potential to control inflammation and improve or stabilize vision (ScienceDirect, 2016).
The use of biologics in uveitis treatment has been reviewed, highlighting the benefits of these agents, including infliximab and adalimumab, in reducing inflammation and improving clinical outcomes (Lippincott Williams & Wilkins).
Conclusion
In conclusion, the management of meibomian gland dysfunction, while important for ocular comfort, does not address the underlying inflammation seen in uveitis. Scientific applications and case studies have demonstrated the benefits of various uveitis treatments.
For instance, local administration of corticosteroids has been shown to be effective in treating anterior uveitis, with a clinical trial illustrating significant improvement in ocular inflammation (S016164201300777X). Similarly, methotrexate, a systemic immunosuppressive agent, has been identified as a viable long-term treatment option for non-infectious uveitis, offering control of inflammation and preservation of vision (1422-0067/16/8/18778).
Literature reviews further consolidate the role of immunosuppressive therapy in achieving remission in uveitis patients, emphasizing the importance of early and aggressive treatment (books.google.com/books?hl=en&lr=&id=vZxqM6cuQI4C). Moreover, biologic agents like anti-TNFα inhibitors have been recognized for their efficacy in refractory uveitis, supported by case studies showing positive outcomes in reducing uveitic flares and improving visual acuity (PMC3808925).
In more complex scenarios, the use of intravitreal implants delivering corticosteroids has emerged as a powerful tool to control intraocular inflammation, with studies highlighting their role in reducing macular edema and improving vision in patients with non-infectious posterior uveitis (S0002939409008824).
Furthermore, the therapeutic landscape of uveitis has been expanded with the advent of new pharmacologic approaches, such as interferon therapy, which has shown promising results in controlling ocular inflammation in Behçet’s disease-associated uveitis (link.springer.com/article/10.1007/s11926-006-0006-6). Case studies have also illustrated the potential of monoclonal antibodies, like adalimumab, for the treatment of various forms of refractory uveitis, thereby improving quality of life for patients by reducing inflammation and preventing relapses (S0161642016307357).
Biologics, as a newer class of therapeutics, have been the focus of numerous studies, with reports confirming their effectiveness in achieving disease quiescence and reducing the burden of corticosteroid usage in uveitis treatment (journals.lww.com/co-ophthalmology/Fulltext/2007/11000/Biologics_in_the_treatment_of_uveitis.8.aspx).
Finally, advancements in pharmacotherapy have been complemented by the development of novel drug delivery systems, such as bioerodible implants, which allow for sustained release of medication, minimizing the need for frequent intraocular injections and improving adherence to treatment regimens (PMC3744776).
While meibomian gland dysfunction and uveitis are distinct conditions, the evolution of therapeutic strategies for uveitis showcases the importance of targeted interventions in ocular diseases, improving clinical outcomes and enhancing patient quality of life.